
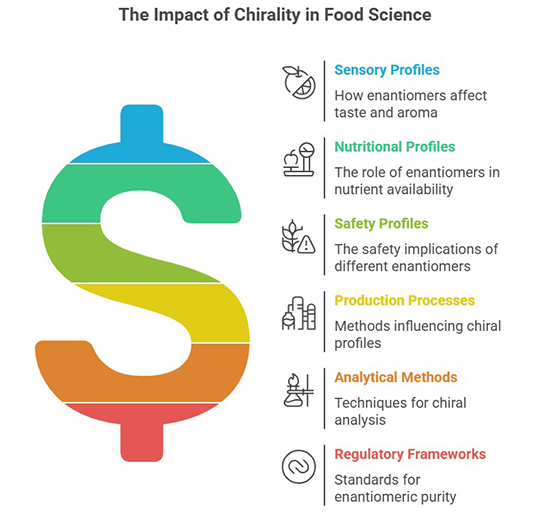
Chirality, the geometric property of molecules existing as non-superimposable mirror images (enantiomers), plays a pivotal role in the sensory, nutritional, and safety profiles of food and beverages. Enantiomeric differences in natural and synthetic compounds—such as amino acids, sugars, terpenes, and additives—can dramatically alter flavor, aroma, bioavailability, and toxicity. For instance, L-amino acids dominate in proteins, while D-sugars form the backbone of DNA, and enantiomers like R-limonene (orange) and S-limonene (lemon) impart distinct citrus notes.
The implications of chirality extend to food safety, where one enantiomer may pose health risks while its counterpart is benign or beneficial, as seen in certain pesticides or preservatives. Production processes, including fermentation, thermal treatment, and enzymatic catalysis, further influence chiral profiles, necessitating precise analytical methods (e.g., chiral chromatography, spectroscopy) for quality control. Regulatory frameworks increasingly address enantiomeric purity, reflecting growing awareness of chirality's impact on consumer health and product authenticity.
This interdisciplinary field bridges chemistry, food science, and biotechnology, driving innovations in sustainable production, flavor engineering, and safety assessment. Emerging research explores metabolic processing of enantiomers, chiral additives in functional foods, and biotechnological applications to tailor enantiomeric ratios. Understanding chirality is thus critical for advancing food innovation, ensuring safety, and meeting regulatory standards.
The following publications delve into these themes, offering insights into chiral analysis, sensory chemistry, toxicology, and industry practices, while highlighting challenges in standardization, detection, and consumer education.